Background:
Blinatumomab, a CD3×CD19 T cell-engaging antibody was developed for immunotherapy in patients with relapsed/refractory B-lineage acute lymphoblastic leukemia (R/R B-ALL) and positive minimal residual disease complete remission. However, high tumor burden is thought able to predict a lower object response rate (ORR) after treatment with blinatumomab monotherapy, so combination treatment regimens are needed to improve the therapeutic effect of blinatumomab. Previous studies have reported the clinical trial results of adding blinatumomab to chemotherapy such as Mini-hyper-CVD and CAM for the treatment of adult or infant R/R B-ALL patients. In this retrospective study, we set out to test the safety and efficacy of single-cycle blinatumomab plus VP scheme in adult R/R B-ALL patients with high tumor burden.
Method:
Adult patients (≥18 years of age) diagnosed as R/R B-ALL with a high tumor burden (blast count in the bone marrow >50%), previously received 1 to 3 lines of chemotherapy, and received single-cycle blinatumomab plus VP were enrolled. These patients were given blinatumomab (9μg/d in d1-d7, 28μg/d in d8-d28), vincristine 2mg/week and dexamethasone (10mg/d in d1-d14, 5mg/d in d15-d28), for a total of 28 days. For patients with Philadelphia chromosome-positive, a BTK kinase inhibitor (TKI), including imatinib/dasatinib/olverembatinib, etc., was administered simultaneously. Eligible patients were treated with allo-HSCT or CAR-T therapy after they achieved remission with single-cycle blinatumomab plus VP. All patients underwent bone marrow examination before and after treatment to determine tumor burden and therapeutic effect. Flow cytometry was used to monitor patients' MRD status, with abnormal cells ≤0.01% defined as MRD-negative. OS is defined as the time from the initiation of the treatment to death for any cause. RFS is defined as the time from achievement of CR to disease recurrence or death after the treatment.
Results:
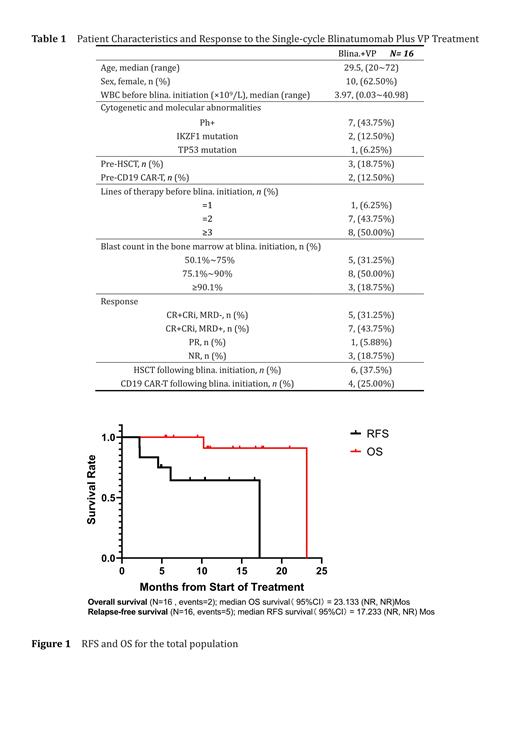
Sixteen patients started this treatment between August 2021 and February 2023. The median age of the 16 patients were 29.5 years (range, 20-72), and their baseline characteristics were shown in the table. Seven of the patients were Philadelphia chromosome-positive. Prior to receiving blinatumomab, all patients had received at least first-line chemotherapy and had refractory or recurrent events. At the end of single-cycle blinatumomab plus VP treatment, 12 patients (75%) achieved objective response (CR+CRi), 1 patient (6.25%) achieved PR, 3 patients (18.75%) did not achieve response, and no patients died during treatment. Of the 12 patients in objective response, 3 patients (18.75%) achieved CR with MRD-negative, 2 patients achieved CRi with MRD-negative, and 7 patients achieved CRi with MRD-positive. Six patients were bridged with allo-HSCT after the treatment, and four patients were subsequently treated with CD19 CAR T cells. With a median follow-up of 11.07 months, the median overall survival was 23.13 months and the median RFS was 17.23 months.
The majority of adverse events during treatment were mild and acceptable. Twelve patients (75%) developed fever, of which nine patients (56.25%) were diagnosed with febrile neutropenia, and eight patients (50.00%) had an infection requiring antibiotic treatment, but none required high-flow oxygen (≥6L/min) or vasoactive agents to maintain blood pressure. Almost all patients (93.75%) had myelosuppression during treatment (neutrophil in peripheral blood <1.0×10 9/L), the median time was 17.5 days (range, 0d~28d). Elevated ALT/AST was observed in only one patient. No above grade 3 cytokine release syndrome (CRS) and any grade of immune effector cell-associated neurotoxicity syndrome (ICANS) were observed.
Conclusion:
Blinatumomab combined with VP resulted in a higher response rate in adult R/R B-ALL patients with high tumor burden and increased tolerability and safety of the treatment.
Disclosures
No relevant conflicts of interest to declare.